Hematopoiesis: Unraveling The Process Of Blood Cell Formation
Hematopoiesis, the process by which blood cells are formed, is a complex and intricate process that takes place primarily in the bone marrow. This process involves the differentiation of stem cells into various types of blood cells, including red blood cells, white blood cells, and platelets. Understanding hematopoiesis is essential for developing treatments for hematologic disorders, such as leukemia, anemia, and thrombocytopenia.
Researchers have made significant progress in unraveling the mechanisms of hematopoiesis in recent years. This article aims to provide an overview of the bone marrow and the role of stem cells in hematopoiesis, as well as the differentiation process and the importance of cytokines in regulating blood cell formation. Additionally, we will explore various hematopoietic disorders, their current treatments, and promising new developments in hematopoietic research.
By shedding light on the intricacies of hematopoiesis, this article aims to contribute to the ever-growing knowledge base in this field and inspire further research in the quest for improved treatments and cures for hematologic disorders.
Key Takeaways
- Hematopoiesis is the process of blood cell formation primarily in the bone marrow, and hematopoietic stem cells (HSCs) have the ability to self-renew and differentiate into all blood cell lineages.
- The bone marrow microenvironment plays a critical role in regulating hematopoiesis, and cytokines play a crucial role in the regulation of gene expression and cell fate decisions during the development of various blood cell lineages.
- Malignant and non-malignant hematopoietic disorders are characterized by the uncontrolled proliferation of abnormal blood cells or disruptions in the normal production and function of blood cells, and various treatments including chemotherapy, stem cell transplantation, and gene therapy are available.
- Advancements in gene therapy and stem cell research provide hope for targeted and permanent treatments for hematopoietic disorders, but safety concerns and high costs remain as potential challenges.
The Bone Marrow: Where Blood Cell Formation Begins
The bone marrow serves as the primary site for hematopoiesis, where the process of blood cell formation begins and various progenitor cells differentiate into different blood cell lineages. Hematopoiesis is a highly regulated process that involves a complex interplay of different molecular and cellular interactions.
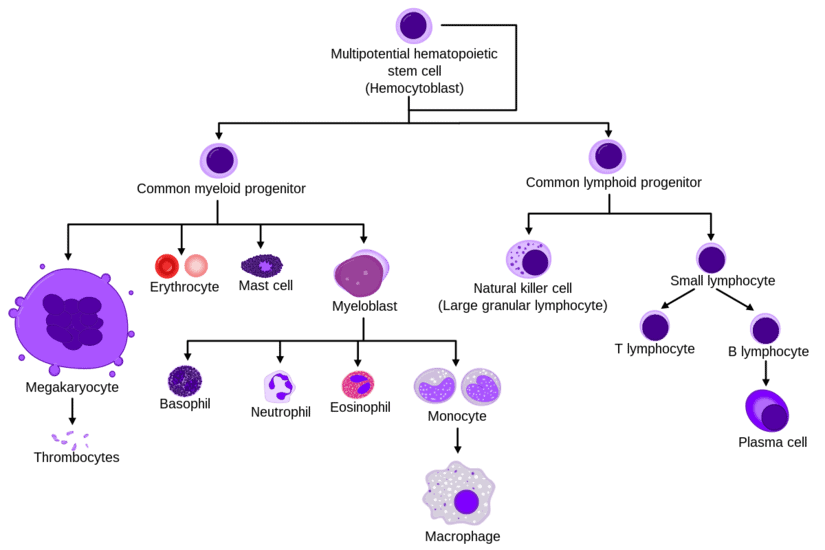
The process starts with hematopoietic stem cells (HSCs), which have the ability to self-renew and differentiate into all blood cell lineages. HSCs give rise to progenitor cells, which undergo several rounds of division and differentiation to produce mature blood cells.
The bone marrow microenvironment plays a critical role in regulating hematopoiesis by providing the necessary signals and support for the survival and differentiation of blood cell progenitors. The bone marrow stromal cells, including osteoblasts, endothelial cells, and mesenchymal stem cells, secrete various cytokines and growth factors that regulate the proliferation and differentiation of HSCs and progenitor cells.
Additionally, the bone marrow extracellular matrix provides a physical scaffold for the attachment and migration of blood cell progenitors. The complex interactions between the bone marrow microenvironment and blood cell progenitors are essential for maintaining the homeostasis of the hematopoietic system.
The Role of Stem Cells in Hematopoiesis
Stem cells play a crucial role in the generation and maintenance of all blood cell types in the human body. These cells are responsible for self-renewal as well as differentiation into various blood cell lineages.
The process of hematopoiesis begins with the differentiation of hematopoietic stem cells (HSCs) into multipotent progenitor cells (MPPs), which can further differentiate into various lineage-restricted progenitor cells. These progenitor cells then undergo several rounds of division and differentiation to finally form mature blood cells.
The ability of HSCs to differentiate into various blood cell types makes them a valuable source for regenerative medicine and gene therapy. Researchers have been exploring ways to use HSCs to treat various blood disorders such as leukemia, sickle cell anemia, and thalassemia.
The understanding of the mechanisms that regulate HSC self-renewal and differentiation has led to the development of various in vitro methods to expand and differentiate HSCs. However, more research is needed to fully understand the complex process of hematopoiesis and to develop effective therapies for blood disorders.
Differentiation: How Blood Cells Take Shape
Differentiation of hematopoietic stem cells leads to the formation of various blood cell lineages through a series of complex molecular and cellular events. This process is tightly regulated by a network of transcription factors, signaling pathways, and epigenetic modifications that control gene expression and cell fate decisions. The differentiation process can be divided into several stages, each characterized by the expression of specific markers and the acquisition of distinct functional properties.
The first stage of differentiation involves the commitment of hematopoietic stem cells to a particular lineage, such as myeloid or lymphoid. This decision is influenced by extrinsic signals from the microenvironment, such as cytokines and growth factors, as well as intrinsic factors that determine the cell’s developmental potential. Once committed, the cells undergo a series of divisions and maturation steps that result in the formation of mature blood cells with specialized functions. The table below summarizes the major stages of differentiation and the key markers associated with each lineage.
| Lineage | Stage | Markers |
|---|---|---|
| Myeloid | Common myeloid progenitor | CD34+, CD38+, CD123+ |
| Granulocyte-macrophage progenitor | CD34+, CD38+, CD123-, CD45RA- | |
| Megakaryocyte-erythroid progenitor | CD34+, CD38+, CD123-, CD45RA+ | |
| Granulocyte | CD15+, CD33+, CD66b+ | |
| Monocyte | CD14+, CD11b+, CD64+ | |
| Lymphoid | Common lymphoid progenitor | CD34+, CD10+, CD45RA+ |
| B-cell | CD19+, CD20+, CD22+ | |
| T-cell | CD3+, CD4+ or CD8+ |
Overall, the differentiation of hematopoietic stem cells is a complex and highly regulated process that gives rise to the diverse array of blood cell types required for normal immune function and tissue homeostasis. Understanding the molecular mechanisms that control this process is essential for developing new therapies for blood disorders and improving our ability to manipulate stem cells for regenerative medicine applications.
The Importance of Cytokines in Hematopoiesis
Cytokines play a crucial role in the regulation of gene expression and cell fate decisions during the development of various blood cell lineages. They are signaling molecules that affect the proliferation, differentiation, and survival of hematopoietic stem cells (HSCs) and progenitor cells.
Here are some of the ways cytokines influence hematopoiesis:
- They instruct HSCs to differentiate into specific blood cell lineages, such as erythrocytes, leukocytes, and platelets.
- They promote the survival of hematopoietic cells by preventing apoptosis, a form of programmed cell death.
- They stimulate the proliferation of hematopoietic cells, allowing for the production of large numbers of blood cells in response to an infection or injury.
- They regulate the migration of hematopoietic cells from the bone marrow to other tissues, such as the spleen and lymph nodes.
- They modulate the activity of other cytokines and growth factors, creating a complex network of signaling pathways that control hematopoiesis.
Overall, the importance of cytokines in hematopoiesis cannot be overstated. Without these signaling molecules, the development and maintenance of the blood cell system would be severely compromised, leading to a range of diseases and disorders.
Hematopoietic Disorders: When Blood Cell Formation Goes Awry
Various diseases and disorders can occur when the normal balance of blood cell production and function is disrupted. These disorders can be broadly classified as either malignant or non-malignant.
Malignant hematopoietic disorders are characterized by the uncontrolled proliferation of abnormal blood cells, which can then infiltrate and damage healthy organs and tissues. Examples of malignant hematopoietic disorders include leukemia, lymphoma, and multiple myeloma.
Non-malignant hematopoietic disorders are characterized by disruptions in the normal production and function of blood cells. These disorders can be further classified based on the affected cell lineages. For example, anemia is a non-malignant hematopoietic disorder that affects the erythroid lineage, resulting in a decreased production of red blood cells. Other examples of non-malignant hematopoietic disorders include thrombocytopenia (a decreased production of platelets), neutropenia (a decreased production of neutrophils), and myelodysplastic syndromes (a group of disorders characterized by abnormal cell morphology and function in the bone marrow).
Treatment of hematopoietic disorders varies depending on the specific disorder and can include chemotherapy, radiation therapy, bone marrow transplantation, and supportive care.
Current Treatments for Hematopoietic Disorders
Treatment options for hematopoietic disorders have advanced significantly in recent years, allowing for more personalized and effective approaches to managing these conditions.
One of the most commonly used treatments is chemotherapy, which involves the use of drugs to kill cancer cells. Chemotherapy can be administered orally, through injections, or intravenously. This treatment is effective in managing leukemia, lymphoma, and multiple myeloma. However, it also has side effects such as nausea, hair loss, and fatigue due to the damage it can cause to healthy cells in the body.
Another treatment option for hematopoietic disorders is stem cell transplantation. This involves replacing damaged stem cells in the bone marrow with healthy ones. The healthy stem cells can come from a donor, or they can be harvested from the patient’s own body. This treatment is used for a variety of conditions, including leukemia, lymphoma, and sickle cell anemia.
While stem cell transplantation can be highly effective, it is also a complex and risky procedure that can have serious side effects such as infections and organ damage. Nonetheless, it remains an important tool in the fight against hematopoietic disorders.
New Frontiers in Hematopoietic Research
Advancements in research on hematopoietic disorders have led to groundbreaking discoveries in gene therapy, providing hope for more targeted and permanent treatment options. Gene therapy involves the manipulation of genes within a person’s cells to treat or prevent diseases. In the context of hematopoietic disorders, gene therapy aims to correct genetic mutations that cause abnormal blood cell formation.
One promising approach is the use of chimeric antigen receptor (CAR) T-cell therapy, which involves modifying a patient’s own immune cells to target and destroy cancerous blood cells. CAR T-cell therapy has shown remarkable success in treating certain types of leukemia and lymphoma, and ongoing research is exploring its potential for other hematopoietic disorders.
Another area of research is the use of induced pluripotent stem cells (iPSCs), which can be generated from a patient’s own skin or blood cells and then differentiated into various types of blood cells. iPSCs have the potential to provide an unlimited supply of healthy blood cells for transplantation, reducing the need for donor matching and the risk of rejection.
The Future of Hematopoiesis: Promising Developments and Challenges Ahead
The continued exploration of gene therapy and stem cell research shows promise in the development of targeted and permanent treatments for a range of hematopoietic disorders, but also presents challenges in ensuring safety and efficacy. Gene therapy involves the insertion, removal, or alteration of genes to treat or prevent diseases, while stem cell research focuses on the use of stem cells to repair or replace damaged tissues. Both approaches have shown potential in treating hematopoietic disorders such as sickle cell anemia, hemophilia, and leukemia.
However, there are still challenges that need to be overcome before gene therapy and stem cell research can be widely used as treatments for hematopoietic disorders. One major challenge is ensuring the safety and efficacy of these treatments, as they involve manipulating genetic material and may have unintended consequences. Another challenge is making these treatments accessible and affordable to those who need them, especially in developing countries. Despite these challenges, the continued advancements in gene therapy and stem cell research provide hope for the development of targeted and permanent treatments for hematopoietic disorders.
| Advantages | Disadvantages |
|---|---|
| Targeted treatments | Safety concerns |
| Permanent cures | High cost |
| Potential for personalized medicine | Limited accessibility |
| Potential for reducing healthcare costs in the long run | Limited understanding of the long-term effects |
Conclusion
In conclusion, hematopoiesis is a complex process that involves the differentiation of stem cells into various blood cell types. This process is regulated by cytokines and growth factors that play a crucial role in maintaining the balance between different blood cell lineages. However, hematopoietic disorders can arise when there is a disruption in this delicate balance, leading to the overproduction or underproduction of certain blood cells.
Although there are currently treatments available for some of these disorders, there is still much to be learned about hematopoiesis and the underlying mechanisms that drive it.
New frontiers in hematopoietic research, such as gene editing and regenerative medicine, hold promise for developing novel therapies for hematopoietic disorders. However, these advancements also pose ethical and regulatory challenges that must be addressed.
Furthermore, there is a need for continued research to better understand the complexities of hematopoiesis and to develop more effective treatments for hematopoietic disorders. Overall, the future of hematopoiesis research is both exciting and challenging, and it is vital for researchers to work towards improving our understanding of this vital process.